|
The great brain robbery:
|
|
FOR WEEKS, news services had warned that the Michelangelo virus might wreak havoc on hapless computers around the world that day, March 6th, 1992. Millions of computer users nervously booted up, scrutinizing the sequence of "credits" that flickered across the screen as their PC did its warm-up exercises. Was that a new screenful of characters that flickered momentarily? Was that RAM-reading any different from normal? But their anxiety proved unnecessary. As the day wore on Michelangelo proved to be a dud. Unless tampered with, computer programs instruct computers to behave in totally predictable ways. In fact, they simply cannot behave in any other way, assuming the computer hardware itself is in normal working order. If you are running a word processing program, you know it will wrap words around the end of the line 100% of the time without your having to think about it.
As everybody knows, however, some nasty fiends lie awake at night pondering how to create new sets of instructions, commonly called viruses, that will make your computer do unwanted things. These pariahs of cyberspace know exactly how the virus will affect your computer once it has gained access. Viruses that change the behavior of computers in predictable ways are an invention of the technological 1980s. God beat us to it by a long shot, having created living analogues of computer viruses, not for the purpose of causing havoc in nature but to demonstrate yet again the endlessness of His ingenuity. As sci-fi as it sounds, He has created some little parasitic creatures that, once they have gained access into a living computer of the right kind, will change its behavior, turning the poor host into a kind of zombie. Parasite expert, Janice Moore, put it this way: One of the most familiar literary devices in science fiction is alien parasites that invade a human host, forcing him to do their bidding as they multiply and spread to other hapless earthlings. Yet the notion that a parasite can alter the behavior of another organism is not mere fiction. The phenomenon is not even rare. One need only look in a field, a lake or a forest to find it (1984, p. 108). These aliens occupy part of a broad spectrum of parasites that have pronounced effects on their landlords; the name of the game is "manipulation". These guests don't take complete control of their hosts, like mutineers on a ship, but they can commandeer their normal functioning in some limited manner or modify their behavior in specific and intriguing ways, or both, so as to enhance their own survival or, most commonly, to boost their chances of being transmitted. Such manipulation of hosts by parasites was first suspected in the 1930s, but no hard data emerged until van Dobben discovered in 1952 that fish retrieved from cormorants' guts had a higher rate of infection than fish hooked by fishermen; something about the infected fish made them more attractive to the birds. Little further progress was made until the 1970s, when laboratory experiments proved that amphipod crustaceans infected by certain worms behaved strangely, making them almost irresistible to ducks (Thomas and others 2002, p. 186). In 1982, Richard Dawkins coined the phrase "extended phenotype" to describe the phenomenon, noting that the genes of one organism (the parasite) could influence the phenotype (form, physiology, or behavior) of another (its host). Interest in the phenomenon has accelerated ever since; an entire 2005 issue of the prestigious journal "Behavioural Processes" was devoted to the topic. Intrigued? Buckle your seatbelts and read on. This article will provide a sampling of amazing interactions, studied both in the laboratory and the field, between squatter and landlord, between enslaved host and tyrannical parasite. We hope that, unlike Richard Dawkins, you will recognize the endless inventive genius of God on display. The more that is learned, the harder-pressed experts seem to be to explain what is really going on. They have learned, however, that the phenomenon is widespread and that altered behaviors range in magnitude from "slight shifts in the percentage of time spent in performing a given activity to the production of complex and spectacular behaviors" (Thomas and others, p. 186). Naturally, our survey will be biased towards the latter. Host manipulation
Stop and think. You are a parasite firmly lodged inside a pill bug and the irresistible urge has come upon you to get inside a starling to complete your life cycle.1 How do you get there? You have one big problem - your pill bug friend loves dank, dark places, and spends all day long dozing under logs or in rocky crevices. While Sam Starling is plying his trade, Pete your pill bug is cozily napping under a log. Or you are cooped up inside Horst the horned grasshopper and cannot consummate your purpose in life until you find water in which to join a mating frenzy. What to do? Enter stage left. host manipulation. You push Pete into the open; you lead Horst to water. The name of the game is to hijack your host and force it to acquiesce to your demands. To speak of manipulation raises conceptual difficulties; how does one define "behavioral manipulation"? Some parasites change the behavior of their hosts through pathogenic effects, that is, by making them sick and thus less capable of evading their fleet-of-foot predators. Such debilitation of the host increases the lodger's chance of moving on in life and thus can surely be considered a form of exploitation geared to enhance the lodger's survival; but can it be called manipulation? Examples of this kind abound. Elk can be so weakened by the ravages of lungworms that they make little effort to flee from attacking wolves, the worm's final host. Some fish infected with larvae of tapeworms or flukes swell up and become more conspicuous or more buoyant, making it harder for them to remain incognito or to dash to safety when the shadow of a bird passes overhead. In many cases, altered behavior consists merely in increases or decreases in activity (such as moving, eating, or sleeping), or a change of hangout. Experts on the subject sometimes get bogged down in philosophical discussions about the validity of using the term "manipulation" to describe such infection-caused changes in host structure and habits. They argue that unless one can prove that the guest "deliberately" commandeers host physiological or nervous system functioning it should not be considered manipulation but merely an incidental byproduct of harboring unwanted spongers. Thus you come across titles such as, " Host manipulation by Ligula intestinalis: accident or adaptation? ", or " Parasite-induced change in host behavior of a freshwater snail: parasitic manipulation or byproduct of infection?" Compounding the problem, even experts often find it hard determining exactly how the changes in host behavior are brought about. Consequently, we often don't know whether the villains are brain robbers or merely cheap thugs - whether a changed behavior is due to tampering with the wiring or biochemistry that controls behavior or whether it's due to the kind of pathogenic effect just mentioned. Some systems appear straightforward enough. Larvae of the nematode Tetrameres americana settle into the muscles of certain grasshoppers making them less jumpy and so far more likely to be snapped up by their final host - a chicken. Sheer thuggery, for sure. Other cases are less clear. Fluke larvae living in Hawaiian corals, their intermediate host, "manipulate" the coral in such a way as to increase predation by butterfly fish, their final host, on the corals. Increased predation is a simple function of a new attribute of infected coral; contaminated polyps "cannot perform an important polyp behavior - retraction - and are left exposed to predators" (Moore 2002, p. 45). Is the inability of the fleshy polyp to retract into its protective skeleton due to simple muscle fatigue or sophisticated scrambling of nerve connections by its lodger? It's hard to say. Certain ants that serve as intermediate hosts for a tapeworm that infects woodpeckers will not flee when the bird breaks into their dark retreat in a branch. Have the ants been rendered sluggish by deterioration of muscle tone or by a smart attack on their nervous system? If an infected fish swims closer to the surface is it caused by changes in the control network or merely to gain access to higher oxygen levels because its large parasite drains its oxygen supply? Again, it's hard to say.
Some of the aberrant behaviors that infected organisms display may result from nutritional distress or metabolic disruptions brought on by the parasite. Hosts that are starved of nutrients by their lodgers may become sluggish and so are less responsive to danger than normal. By the opposite token, some hosts may make a superhuman effort and increase feeding activity in order to compensate for the drain on their resources, making themselves more conspicuous in the process. The changed behavior is then an incidental side effect of parasitism rather than being caused by direct manipulation of the central nervous system (CNS). So the "real" cause can be very hard to pin down. In spite of the kind of uncertainties just surveyed, other cases force the conclusion that genuine manipulation is at work. As Robert Poulin says in the bizarre case of a fluke that reaches sheep via ants, "Who could believe that the grass-climbing behavior that Dicrocoelium triggers in its intermediate host is a coincidental pathological consequence?" (1995, p. 1371). The bottom line is this: God can think up unlimited strategies for achieving a particular goal or solving a particular problem. Whether the immediate cause of changed behavior is pathological or manipulative is, in one sense, irrelevant. However, this article specifically focuses on manipulation, and we will try hard to keep that in mind. A miscellany of manipulationThe ultimate purpose of manipulation of hosts is to enable parasites to complete their life cycle. Systematically categorizing all the various forms and functions of manipulation lies outside the scope of this offering. First we'll describe a few miscellaneous cases after which we will turn our attention to the recurring theme of manipulation designed to facilitate transmission. In one striking case, a wasp that parasitizes the potato aphid (Macrosiphum euphorbiae) causes the aphid to leave its colony when the wasp grub is ready to metamorphose and to seek out a suitably protected spot for the duration. Some of the aphid hosts entered curled-up leaves at the base of the plant, while others left the plant and searched out concealed sites (Brodeur & McNeil 1989, p. 227). The authors suggest that the wasps enjoy a double-whammy of protection from this behavioral manipulation. First, they would enjoy protection from the negative effects of freezing rain and hail and, secondly, from other wasps that might parasitize the parasite or from predators that would love to eat them. Female wasps of the genus Ibalia use their sense of smell to find fresh drill holes in pine trees formed by ovipositing females of the notorious Sirex wasps, considered by many as the worst nightmare of foresters for the damage they wreak: She then lowers her ovipositor into the hole, drills into the sirex egg, and deposits an egg into the body of the developing sirex larva. By the time the sirex larva hatches and bores into the wood, it has an lbalia parasite larva inside it. The lbalia larva grows inside the sirex larva for several months, then bites its way out and eats the remains of the sirex larva (Fig. 14). It pupates in early summer, turns into an adult and chews its way out of the wood leaving a small round hole (Forest and Timber Insects in New Zealand, Scion, No. 47). Ibalia's escape from its woody cage is facilitated by its capacity to alter its host's normal behavior. Ibalia grubs have weak jaws and are incapable of sustained chewing through wood. Sirex grubs normally bore deep into the wood, but when infected by Ibalia, they bore towards the surface thereby helping their freeloader escape and go searching for more Sirex grubs to infect (Denton 1985, p. 223).
In another case in which a wasp, Ichneumon eumerus, alters its host's behavior to protect itself from the life-threatening dangers of inclement weather the victim is a spider. The culprit, a parasitoid (a parasite that kills its host at some stage), hijacks its host spider's web-making program, forcing it to change its spinning routine. Instead of weaving the usual insect-grabbing net, the blighted arachnid spins a wasp-saver: On the evening that it will kill its orb-weaving spider host, the larva of the. wasp Hymenoepimecis induces the spider to build an otherwise unique "cocoon web" to serve as a durable support for the wasp larva's cocoon (Eberhard 2000, p. 255). The new web provides an effective rain shelter for the metamorphosing wasp pupa suspended in its cocoon below. (Studies on Hymenoepimecis species show that the pupae are highly vulnerable to heavy rain.) In order to serve effectively as a shelter, rough enough is definitely not good enough; the new web must be just right. After describing some of the differences between old and new webs, Eberhard says, "A single 'mistake'. could be disastrous for the wasp larva. These differences between cocoon webs and normal orbs make the cocoon web a stronger, more durable support for the wasp's cocoon". Web-building behavior in spiders is mysterious enough in its own right. How a grub can alter the genetically-controlled, stereotyped instinct in the way just described defies understanding. When investigators remove grubs before spinning begins, the spider nevertheless stitches up a wasp-friendly web; the grub has left its instructions behind! Utterly unbelievable. In another wasp case, 24 hours prior to their emergence from their host, wasp grubs inside the tobacco hornworm caterpillar induce it to stop feeding and to reduce locomotion. This behavior, which has been shown to be brought about by interference with the host's nervous system, benefits the grubs by, "preventing the host from feeding on, or dislodging, the wasp cocoons" (Adamo 2002, p. 372). In 2011, researchers at the Universities of Montpelier and Montreal discovered another case of zombie behavior in a manipulated insect: The parasitic wasp Dinocampus coccinellae is no fool. It controls a ladybug, lays an egg in its abdomen and turns it into the bodyguard of its cocoon. Females deposit a single egg in the abdomen of their host, the ladybug, and during larval development (around twenty days) the parasite feeds on the host's tissues. Then, the wasp larva breaks out through the ladybug's abdomen, without killing it, and begins spinning a cocoon between the ladybug's legs. The ladybug, partially paralyzed, is forced to stand guard over the cocoon (Parasitism: Wasp Uses Ladybug as "Zombie Bodyguard"). Experiments showed that this method proves very successful in protecting the wasp from predation during the cocoon stage. One of the amazing, if not unique, features of this relationship is that the manipulation occurs after the parasite has left the host. Now that's some neat trick.
Horsehair worms, known technically as nematomorphs, are extremely long, threadlike creatures found in water; lengths of 14 inches (36 cms) or more are typical while they rarely exceed one millimeter in diameter. While males commonly swim actively or crawl about by whiplike motions females tend to laze their lives away. Horsehair worms develop fully from larva to adult inside a single host; no intermediate hosts are involved. The female lays her eggs in the water in long strings; these hatch into short-lived larvae that swim around until they come into contact with a suitable host such as an insect or spider that has either visited the water for a drink or has fallen in momentarily. Survival of the larva hangs in the balance; the insect must extricate itself from the water and return to life on land or the larva is doomed. Development into the adult form takes place entirely inside the host.
To complete the life cycle, the trapped worm must break out to freedom from its prison in order to mate and lay eggs. In this case, freedom requires a return to the water because the adult worms can neither survive nor reproduce on land. But how can the cocooned worm get back to the water? No problem for divine wisdom. The Great Designer built a wondrous trick into the horsehair - it leads its host to water. Most hairworm hosts are about as fond of water as the artful dodger. In their charming article, "Do hairworms (Nematomorpha) manipulate the water seeking behavior of their terrestrial hosts?", Thomas and others report on two years of observations of crickets coming out of the woods and heading for a nearby swimming pool. Upon reaching it, they would plunge in; very soon after a strand of "living hair" would be seen snaking and writhing its way out through the cricket's skin. Systematic follow-up experiments showed definitively that infected crickets were positively attracted to the pool; 95% of the crickets found around the pool were infected compared with 0% (in one year of studies) and 15% (in the other year) collected in surrounding forest (2002, p. 358). Of uninfected crickets found around the pool, only 13% entered the water, while 48% of infected individuals took the plunge within 15 minutes of arrival. The investigators report that, Once infected crickets encounter water, there is an important behavioral difference compared with uninfected individuals. Crickets harboring a worm often jumped into the water whereas uninfected crickets most of the time were reluctant to enter it. Whether infected crickets are attracted by the liquid, or whether they simply do not perceive the danger linked to the presence of water. is not currently known (p. 360). How do the worms do it? That is the question investigators are furiously seeking to answer.
Another spectacular example of manipulation is found among strepsipterans, "the most enigmatic order of insect" (Watanabe 2004, p. 383). These weird insects, the adult males of which look a little like tiny beetles, parasitize other insects. The worm-like female spends her entire life trapped inside the host insect. When mature, a tiny part of her front end protrudes from between the body armor of the host. Free-flying males find and mate with the female, following which she broods the fertilized eggs until they hatch and develop into tiny, mobile larvae known as triungulins which, when ready, escape to the outside to look for a suitable host in which to complete their life cycle. One species, Xenos vesparum, parasitizes a certain wasp. Just before the strepsipteran breaks through its epidermis, an infected wasp will do something it normally doesn't do - it leaves the nest and seeks out other infected wasps which have joined a growing throng in large aggregations. The gatherings, which are found between July and mid-August, become de facto mating aggregations for the strepsipterans as males emerge and mate with any females which have recently protruded their anterior ends. These gatherings appear to be essential to the strepsipterans' success for a simple reason; these wasps exhibit low levels of parasitism (Beani 2006, p. 568), meaning that any male strepsipterans that emerge in the wasp nest will have a jolly difficult time of it finding a female. The wasps do the job for the parasites by socializing with other infected wasps, making it much easier for boy to meet girl. When the orgy is over, The wasps that house the female parasites then overwinter. During this time, young develop within the viviparous female parasite. When the first instars of the parasite emerge, the host wasp dies-after an extended life span (Watanabe 2004, p. 384). Here we have an instance of a parasite both changing its host's behavior and of enhancing its longevity. Standard evolutionary explanations of origins simply cannot account for such complex interactions.
Then you have the intriguing case of the nematode that lodges in the abdomen of certain tree-dwelling ants on Barro Colorado Island, Panama, and makes them swell and turn a conspicuous, garish red. Investigators believe that certain fruit-eating birds mistake the little balls for fruits of Hyeronima and snap them up like children grab at candy. Adding to the conspicousness of the infected ants is a changed in their behavior. Infected individuals take on an erect stance and bob or flag their colorful gasters around, a behaviour not noted in any other species of ant. This system is very sophisticated; infected worker ants remain in the nest doing housekeeping duties until their resident aliens have reached maturity and are ready to pass to the next host. Only then do they venture out and bob their bods around in the open (Yanoviak and others 2008, p. 543). We shall now consider some examples of host manipulation geared to enhancing the parasite's chances of moving on in life. Finding the right houseFor parasites with wings - wasps and flies for instance - completing the life cycle presents no logistical problem; all they have to do is fly to their next host and lay an egg. Of course, finding their victim may require considerable hunting skills, while placing the eggs in precisely the right spot entails awesome precision, but that's another story. For others it's a different story; wingless protozoans (single-celled animals), non-mobile plant parasites, and various worm-like parasites known collectively as helminths, have extremely limited powers of locomotion and so must find other ways of moving on. These creatures almost universally employ a simple yet effective strategy for increasing the chances of latching onto the next host: massive reproductive potency. Eggs or larvae are produced in such prodigious numbers that a small number will come in contact with target species by chance. "At the level of individuals, the encounter between an infective stage. and its target can be due to many undetermined causes, which are usually referred to as 'chance'" (Combes 1991, p. 970). Ralph Buchsbaum's well-known primer on invertebrates says this: Passive modes of transport are very hazardous. The chance of having the eggs or the larvae eaten by the right kind of host at the proper stage in the life cycle is very small. Only parasites which produce enormous numbers of potential young can survive this kind of life-cycle. It is not surprising, therefore, that most [helminth] parasites seem to live only to reproduce, the reproductive organs occupying most of the animal's body (1948, p. 160).
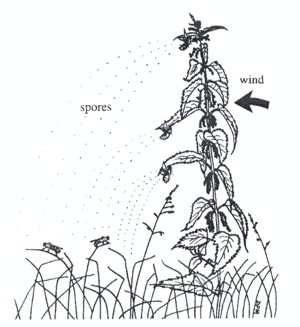
Imagine a fluke egg hatching into a larva whose life cycle requires lodging successively in a snail, a rabbit, and finally in a fox. Say that one in a hundred hatchlings successfully finds a snail, of which only one in a hundred reaches a rabbit of which only one in a hundred ends up on Monsieur Renard's menu. You can do the arithmetic. Yet observations show that infection rates are often quite high. In severely affected regions, almost one hundred percent of the population of some animals can be infected by a particular parasite. Individuals can be shockingly infected; sheep have been found to contain up to 50,000 flukes in their liver. Leaving aside the likelihood that something has gotten out of balance in such cases, the point remains that many parasites have a system for beating the odds that would make the shrewdest gambler drool. Until recently, biologists were largely unaware of aiding-and-abetting strategies. They assumed that producing numbing numbers of crap-shooters was almost the helminth's sole ticket to survival. No longer. They have discovered an alternative basic stratagem to high reproductive capacity for enhancing the probability of transmission: investing resources in quality, rather than quantity. Parasitologists now realize that a host of ingenious strategies based on this one theme exists to increase the chances of parasites finding their way "downstream" from egg to intermediate hosts to final host. Free-living larval stages entrusted with the hazardous mission of tracking down and penetrating the next victim display some refined techniques to help them succeed. Some are programmed to erupt from their waterborne host at the time of day when their next host is most likely to be in the vicinity. Varying instinctive responses to light and gravity lead some larvae to find their next host lurking under a rock on the bottom and others to find them swimming around near the surface. Many cercaria larvae of flukes follow the scent of specific chemicals in mucus trails left by snails that serve as their next host. Larvae that use active search techniques tend to have a short life span of about 24 hours, their reserves of energy being committed to locomotion, while those that depend on being fortuitously swept into the waiting jaws of their next host, such as a barnacle stuck to a rock, float passively in the water and allocate their energy reserves to staying alive for up to five days (McCarthy and others 202, p. 915). Such strategies employed by free-living larvae to find their next host attest to the genius of the Creator. But let us now return to the topic - manipulation of hosts by infective agents trapped inside them. One expert sums up the strategy this way: "According to the manipulation hypothesis, a parasite may alter the behavior or physiology of its host in order to promote its own transmission" (Brown and others 2001, p. 519). The method of manipulation varies greatly, but its aim remains invariably the same - to ensure the infected intermediate host is in the right place at the right time, and, as we are about to see, in some cases in the right orientation, to enable its lodgers to successfully change horses on their life's merry-go-round. Climb every mountainOur first case of a lodger bending its host's will to assist transmission involves not an animal larva doing the manipulating but a fungus! Numerous cases can be found in nature in which insects infected by a fungus climb vegetation and die in exposed positions, a phenomenon frequently referred to as "summit disease" (Maitland 1994, p. 187). Investigators have found that in at least one case involving a fungus (Entomophthora muscae) and a fly (the dungfly, Scatophaga stercoraria), the fungus directly manipulates the behavior of its host, causing the dungfly to perch abnormally and in a highly specific manner (p. 188).
The deal is this. Maitland's team carefully studied the positions of fly cadavers and almost-dead flies (I'm not pulling your leg; but before you laugh, remember that God bothered to make it this way) clinging to flower spikes and leaves of thistles and nettles to determine whether any patterns of orientation would be uncovered. Did those clinging to flower spikes face up or down, and those clinging to leaves face inward or outward? Did they perch high or low on the plant? Were they consistently oriented in a particular compass direction? The data was compared with the positions of live perching flies. The results showed a distinct pattern of perching by dead flies that was different from perching habits of live flies. Integrating the results from two different sites showed that dying flies chose specific heights above ground for departing this world and specific orientations on flowers and leaves that differed from the positions and orientations of live flies. Dead flies often faced inward or downward while living ones faced outward or upward when perching. Dead flies were oriented generally at a given compass direction while live ones cavorted carelessly. Dead flies clung to the underside of leaves while live ones sat with dignity on top. Most interestingly, live flies were found about a quarter of the height from the ground as their departed comrades. Although Maitland admits that one can only speculate on the "adaptive significance" (read "purposeful design") of site selection for entering rigor mortis, nevertheless, "all the described features strongly point towards efficient parasite transmission" (p. 191). Hanging on the underside of a lead provides protection from rain, while the higher local humidity favors spore formation by the fungus. The angle of perching increases the distance between fly body and the leaf providing a larger space for spores to be propelled into the air without crashing into the leaf. (Spores are gluey for enhancing sticking to a fly; you don't want them to get stuck on a leaf.) The orientation of the body also ensures that the wings will not obstruct the path of forcibly ejected spores. In particular, their determination to die high and at a given compass orientation relative to prevailing winds strongly suggests that the flies were trying to assist the effective spread of fungal spores inasmuch as these factors "are likely to direct conidia [spores] clear of the plant". Now that's one smart fungus! You and I may find it hard to be stirred by the thought of dungflies seeking just the right spot to die, but surely even the most impassive of souls cannot help but marvel at the attention God paid to detail for the effective perpetuation of a fungal species! Mutations and natural selection? Bah, humbug. Take me homeThe biggest obstacle faced by infective animal larvae released into the big wild world to fend for themselves is the gauntlet of distance separating consecutive hosts. How can these tiny packages of life successfully negotiate the gulf of separation and find the next host? One way is to make your current host change its habits and move closer to where the next host hangs out. (As of 1991, no cases had been reported where the parasite is able to extend its influence from afar to tempt its downstream host to come towards it. [Combes, p. 872]). With that thought in mind, consider the case of marine snails that are induced by their lodgers to stray from the strait and narrow. The fluke, Gynaecotyla adunca, completes its life cycle in shorebirds. To reach them it must first pass through two intermediate hosts, a marine snail and a small shrimp-like creature called a "beach flea". Trouble is, the snail lives down in the water while the fleas live higher up on the beach. Solution? Smash your snail's comfort zone and drive it towards the hills: . parasitized snails exhibit an abnormal taxis3 toward the beaches and sandbars and are found high on the shore during low tides (Combes 1991, p. 871). There the cercariae break out of the snails in response to various stimuli, track down and penetrate beach fleas which have journeyed down to water's edge at low tide. Of interest, cercariae of this species of Gynaecotyla have a reduced tail and so swim very poorly, while those of other species in the same genus which must find their next host in water swim proficiently. Horses for courses. Jennifer Ackerman adds color to the story: The larvae gobble up the mud snail's glands and then somehow prod the snail to leave its customary habitat and move to the higher ground traveled by beach fleas. There they emerge from the snail's body and enter the body of a flea. When a shorebird eats the flea, parasite and all, the larvae mature in the bird's gut and produce eggs, which are released with the bird's feces onto the flats. The cycle then begins anew (p. 90).
The larval parasite must steer the snail in precisely the right direction; infected snails must crawl up-beach for the system to work. Should the parasite make a manipulation error, the snail could end up crawling around in circles, heading for Davy Jones's locker, attempting to burrow to China or to stretch to the moon. How could such a tiny mote of nothingness become so clever as to take over another creature's mind? We can't do it! Neither luck nor selection pressure is involved here; it's clearly a case of brilliant planning. Exactly what goes on in the snail's pea-brain to alter its behavior is, as with nematomorphs and crickets, under intense investigation. Please eat meAs with the case just mentioned of the beach flea being eaten by a shorebird, many parasites make their passage from one host to the next host (usually from intermediate to final) by trophic means; in non-technical parlance, the downstream host eats the upstream host.2 In these cases, manipulation involves making the upstream host more likely to be eaten by the parasite's saving grace, the downstream host. Examples abound, each one displaying subtle nuances of the basic theme. In one case the intermediate host, the beetle Tenebrio molitor , enjoys an increased lifespan of up to 40% when haunted by early stages of the rat tapeworm, Hymenolepis diminuta (Hurd and others, p. 1749). The value of this effect to the parasite seems plain enough - the longer the beetle lives, the greater is its chance of being eaten by the worm's final host, a rat. How the lodger is able to increase its host's lifespan is most interesting; it blocks its host's reproductive cycle, saving the host from having to do the hard work of making eggs. Hurd continues: "By inducing a flow of nutrients from gametes [eggs and sperm] to soma [body], resources will be diverted away from a non-essential process to support the basic metabolic requirements of the parasite and its host". Since the tapeworm larva doesn't use up all the saved nutrients the host is healthier and lives longer. (Note that in this case the parasite is not manipulating its host's behavior but its physiology.) The marine snail, Littorina saxatilis, is parasitized by the larvae of three different species of fluke. At least one of the three, Microphalus piriformes, induces its host to, "climb higher on rocks and remain there, thus making them more vulnerable to predation by the gull definitive [final] hosts at gull breeding colonies" (McCarthy and others 2002, p. 911). Experiments have verified this behavioral modification method. When both infected and uninfected snails were placed on top of rocks, the uninfected ones retreated to safer positions while the infected ones did not. As another example of the power to change its host's behavior, consider the single-celled creature known as Toxoplasma gondyi. This mote of life enjoys the hospitality of a rat before reaching its final host - a cat. Normally, self-respecting rats avoid cats like the plague. Their discriminating sense of smell can pick up cat urine very effectively, initiating a fleeing response. Toxoplasma cleverly not only dims a rat's wariness but even reverses the normal rat distaste for cat urine - an infected rat seems to develop a fatal attraction to the pee; you can guess the rest. Another single-celled parasite, Sarcocystis rauschorum, lives in the snowy owl as an adult, passing its juvenile phase in lemmings. Infected lemmings significantly increase their exploratory activity into new territories. They also show a decrease in wariness, spurning danger as if rendered invisible to the owl. These behaviors seem geared to increasing lemming vulnerability.
In one of the most oft-repeated cases, flukes that inhabit the gut of some European birds have an amazing trick up their sleeve to increase the chances of the intermediate host being eaten by the birds. Larvae infest snails of the genus Succinea, which normally hide in leaf litter as soon as dawn approaches. Infected snails continue to wander around much longer (Attenborough 1990, p. 182). Birds don't have to be early to chance upon these late snails. In the early hours of the morning, a parasite migrates into each of the snail's antennae, squeezing its way in and stretching the wall so that the brilliantly-colored parasite can easily be seen inside. Soon it begins to pulsate at a rate of about 70 times per minute, creating a kind of barber's-pole effect. The snails position themselves on the undersides of leaves with the pulsating antennae protruding beyond the leaf margins in a highly conspicuous manner. The birds then complete the story by pecking off the poor snail's infected tentacles and swallowing them. (Some parasitologists believe that the reason for such peek-a-boo behavior is that the snails are too big for small songbirds to eat, but birds may be induced to peck off a small portion from an invisible larger whole.) The only problem with this particular scenario is that nobody seems sure whether or not anybody has actually witnessed a bird attack said snails (Moore, p. 54) or even whether birds are drawn to the pulsating beacons - what appears conspicuous to humans might not be on a bird's list of prey cues. If birds do actually respond, we have here a case of both morphological (pertaining to form) change and behavioral manipulation of the host for the purpose of aiding transmission. In a variation on this theme, a species of fluke causes its ant host to circle aimlessly on rocky surfaces in open areas. The ants also fail to retreat to their nests as autumn approaches. In addition, infected ants swell up and take on a striped appearance due to exposure of joints in the distended abdomen. Workers know that the final host of this species of worm is the western robin; who can resist concluding that the unusual behavior and form of infected ants renders them more conspicuous to patrolling robins? Climb every grass bladeOnce upon a time a sick sheep passed some pernicious eggs in its feces. Soon thereafter a snail crawled over the offended spot and, in the course of fulfilling its gustatory duties, swallowed some poo along with a number of the eggs. Alack and alas, the eggs were not nutritious little balls of food, as the snail had thought, but packets of potential parasites, the liver fluke Dicrocoelium.
Upon reaching the snail's gut, the insidious little eggs sprung open, giving birth to a brood of miracidia. In no time at all, these tiny shock troops bored through the gut wall and, by means not fully understood, forced their way into the snail's digestive gland. Here, over the next three months, the wee intruders went through a series of magical transformations. Upon some mysterious cue they made their way to the breathing pore of the snail where they were packaged up by the snail into tiny mucus slime balls containing about 500 larvae and then, when the temperature fell, were "sneezed out". Sound like a fairytale? It's not, and it gets even more fantastical. Certain ants, upon discovering these slime balls, carry them willingly but unwittingly back to their nests. Here, worker ants pounce on them and gorge themselves silly. If only they knew! Insidious things shortly come to pass; while most of the infective specks remain in the body cavity, one - the "brain worm" - invariably succeeds in making its way to the nerve network under the mouth (the subesophageal ganglion, or SEG) where it encysts between the nerves that serve the mouthparts. In the course of time, infected ants find themselves irresistibly drawn to Mount Grassblade. Urged on by their inner guests, up they go, climbing to the blade's tip, mandibles opening and closing as they go. Like mountaineers who linger on the summit to enjoy the view, they decide to stay. As the cool of the day begins to envelop the pasture the ants clamp down their mandibles on the blade where they remain locked tightly, as if in a drunken stupor, as long as the temperature remains low. Here the ants are in an ideal position to be included in the meal of any number of naïve, safely-grazing mammals making the most of the evening or morning cool. The rest, as they say, is history. The infective motes end up as tiny worms infesting the liver. Sadly, the brain worm misses out on the feast, sacrificing itself for the good of its colleagues. In a bizarre twist on the death-grip theme, grass-climbing, mandible-clamping ant behavior is also elicited by a fungus of the genus Cordyceps (Libersat et al, p. 190). The ant dies after scaling the grassy height, after which the fungus sprouts its fruiting bodies through the ant cuticle and releases its spore capsules. These explode as they float downwards and infect the surrounding ground with spores. In a similar case, carpenter ants from Thailand have been found to be infected by the fungus Ophiocordyceps unilateralis. In this case the ants don't climb up a blade of grass, as these ants live in forest canopies. Instead, infected individuals "leave their colonies in the high canopy of tropical forests (greater than 20 m) to bite into leaves within a narrow zone 25 cm above the forest floor" (Hughes et. al. 2010, p. 1). This sort of behavior has an illustrious history. Hughes and his colleagues studied a 48 million-year-old fossilized leaf from Germany that bore scars in precisely the same position and pattern as those produced by living ants, leading them to announce they had found "the first example of the stereotypical death grip". Thorny-headed wormsPerhaps the cleverest of all host manipulators are Acanthocephalans, or thorny-headed worms, a phylum of approximately 1200 entirely parasitic species found in marine, freshwater and terrestrial environments worldwide. At least two species infect deep-sea fish. Acanthocephalans present quite a puzzle to evolutionists because they bear little resemblance to any other organism. Modern evolutionary wisdom, based on molecular analysis, tags them as a sister group to rotifers. Do tell.
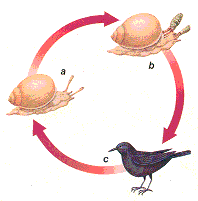
Adult members of this little-known but ubiquitous phylum are generally worm-shaped, of hollow construction, and are characterized by the presence of an eversible proboscis that comes in a dazzling array of shapes and proportions but all of which are armed with recurved spines used to pierce and cling to the wall of the host's stomach or intestines. Many experts believe the proboscis is manipulated by a hydraulic mechanism (Valentine 2004, p. 366). Some species apparently spend time roaming around the host's gut rather than remaining glued to the spot. Adults range in size from less than half an inch, through about eight inches in the case of the common and worldwide rat parasite, Moniliformis dubius, all the way up to two feet long in the case of the aptly-named Gigantorhynchus gigas. Even the longest are only a few millimeters in width. They have no digestive tract and absorb nutrients directly from the surrounding soup by means of an epidermal network of, "invaginations, lacunae, and canals. to produce a vast absorptive surface, between one and two orders of magnitude greater than the areal dimensions of the body" (Valentine, p. 366). Acanthocephalans typically have complex life cycles involving invertebrates as intermediate hosts and the gut of vertebrates, chiefly freshwater fish, as final host. (Some experts entertain the possibility that vertebrates may serve as intermediate hosts in some species.) By contrast with flukes, which always use a mollusk as first intermediate host, thorny-headed worms almost always pass through their developmental stage in a crustacean, such as an amphipod or crab, which becomes infected by swallowing eggs. The egg hatches in the gut and the embryo bores through the gut wall into the body cavity where it finds a cozy place to develop into a cystacanth, a juvenile that has most of the adult features saving reproductive organs. When the crustacean is eaten by the final host (or another vertebrate that is then eaten by the final host), the cystacanth develops into the sexually-reproductive adult. Coupling takes place in the final host's gut and the eggs produced pass out into the great unknown in the host's feces. Transmission from crustacean to final host invariably involves a change in the crustacean's behavior. (No evidence has been found of any physical violence being done to the host's central nervous system.) The case mentioned earlier of a parasite that must pass from pill bug to starling involves an acanthocephalan. The starling excretes the eggs in its droppings which may then be eaten by pill bugs. When the eggs hatch the little invaders proceed to impose their will on the pill bug, making it spend significantly more time in lighted or exposed areas. One species of thorny-headed worm uses mallards as its final host. The intermediate host, a small amphipod, shuns light and therefore normally lives at the bottom of a pond, often in the mud. Since mallards restrict their dives to shallow depths, these amphipods rarely feature on the menu. Infected amphipods, however, cast wisdom aside and head for blue skies whenever they are disturbed. They scud along beneath the surface, sometimes clinging to vegetation or other floating objects, where they are far more likely to be eaten by a surface swimming mallard. These changes in amphipod behavior don't occur until the larva has reached the stage where it can infect mallards; if the amphipod were eaten at an earlier stage, the immature parasite would not survive.
The larva interferes with three crucial amphipod reactions. Sane amphipods prefer darkness, mad ones seek light. Sane amphipods let gravity do its trick, mad ones rebel against it. Sane amphipods duck when they detect a duck; insane ones carry on regardless. Thus, dotty amphipods are much more likely to get eaten. As every housewife knows, cockroaches shun light. However, like mad amphipods, cockroaches infested with another species of thorny-headed worm (Moniliformis dubius, mentioned above) change habits and head for lighted areas where they also become hyperactive. Hyperactive cockroaches that move toward light are less likely to remain hidden, and so are more likely to be caught by rats, their definitive host. A twisted storyWe cannot resist noting a few interesting twists to this whole affair of host manipulation. As we have seen, some embedded larvae incite their hosts to act the fool and get eaten. However, timing is critical; if the host is eaten too soon - that is, before the parasite larva is ready - no good will come of it for either lodger or landlord; the larva must wait until the right time to incite a derring-do attitude in its host. Stop and marvel; a study of a tapeworm-stickleback system in 1993 showed that sticklebacks infected by immature tapeworm larvae behaved even more cautiously than normal (Tierney and others 1993) - exactly the opposite to what we have seen. When the larva is "ripe", however, it twists the control knob in the opposite direction and off goes its foolhardy host looking for trouble. Speaking of such systems, Ponton and others remark that some parasites, ". have independently evolved the capacity to modulate specific behaviors of their intermediate hosts with precise timing and in very subtle ways." to ensure their transmission to their final host (2006, p. 2870). Then you have the hitch-hiking parasites. These are species that, 1) are not themselves capable of changing host behavior, but 2) live in the same area and require the same succession of hosts as other parasites that are capable of manipulating hosts. You guessed it; these clever creatures freeload on freeloaders. Their infective agents actively seek out hosts that are already infected by manipulators. They get all the benefits that their parasitic colleagues get without having to expend any energy in the process. For instance, "the trematode Maritrema subdolum favors its transmission to aquatic birds (definitive hosts) by preferentially infecting gammarids manipulated by the other trematode Microphallus papillorobustus" (Thomas and others 1998, p. 199).
Another possible phenomenon begs thorough investigation; spinoff benefits for numerous predators of infected hosts may play a considerable role in ecosystems as a whole. No natural law states that intermediate hosts rendered more conspicuous by parasites must be eaten by the parasite's special final host. Not all predators serve as a final host for the parasite, meaning that lots of parasites gobbled down with their intermediate host will perish and themselves provide food for some other predator. In experiments in which brook trout were put in with both infected and non-infected guppies; the trout feasted on infected guppies (Combes p. 876). However, brook trout are not the final host for the parasite concerned and so experienced no ill effects. Standing back and looking at the system with a pensive gaze could yield the conclusion that manipulation effects were created for the benefit of the system as a whole, making smaller organisms that have huge populations more amenable to harvesting by top-of-the-line predators. As the plot continues to thicken one cannot help but wonder if future findings will disclose that the manipulated hosts themselves - at least as a species - benefit from being manipulated! This survey barely scratches the surface of this riveting topic. Numerous variations on the theme, some subtle, some drastic, have already been documented in the literature. Who knows what other cases await discovery in the depths of the abyss or the canopies of the Amazon? Time alone will tell. The flip sideWe should not assume from all that has been said that parasites always have the upper hand in dealings with hosts. Not at all; many hosts fight back when parasitized, employing numerous means to rid themselves of unwanted lodgers. Some self-medicate; tiger moth caterpillars in Arizona parasitized by certain fly larvae change eating habits and consume large quantities of a plant containing chemicals which, when ingested in large quantities, usually kill the unwanted lodgers. This behavioral change requires reprogramming their taste buds to make them relish a plant they normally eat very sparingly (Bernays and Singer 2005, p. 476). Some birds rub acid-secreting ants on their skin; researchers believe the acid is absorbed through the skin and irritates or kills parasites. Researchers believe that in some instances self-medication involves eating something analogous to Popeye's spinach - the super food confers greater strength and vitality to the host, giving it a fitness edge in its battle with the unwanted lodger. Some hosts raise their body temperature by physiological means to a point that kills the lodger. Others develop a "behavioral fever", meaning that they seek out warmer places; once again, heightened body temperature kills the parasite. By contrast, bumblebee workers parasitized by certain flies stay away from their nests and seek out colder temperatures, a method that doesn't kill the fly but checks its growth. In a rather fascinating twist on behavioral manipulation, whereas most thorny-headed worms turn cockroach hosts into exhibitionists to the cockroach's hurt, in a few instances worms bring about the opposite result and the infected cockroach shuns light even more rigorously than normal (Poulin 1995, p. 1375)! The bottom line is this: all parasite-host systems work beautifully, thank you, so that both lodgers and landlords survive perfectly well as species no matter whether lodger is subservient to landlord or vice versa. To have any hope of grasping how such a huge variety of host-parasite systems all work so well would require detailed knowledge of an immense number of factors. Investigators with a mathematical bent have modeled many such systems with varying degrees of success. Back in 1980 a seminal, generalized model of host-parasite relations was worked out by Bill Hamilton suggesting that in any given lineage of potential host species the ever-changing set of alleles brought about by recombination during sexual reproduction provides the species its best possible collective defense against parasite virulence. Yet parasites continue to survive in spite of such strategies. In this ongoing, divinely-approved arms race, host species continue to survive and thrive; so do their lodgers.
Millions of years is a long time. However, no matter how much time is available, the notion that such sophisticated and complex strategies of aggression and defense found in parasites and hosts - all finely-tuned and delicately balanced to the highest possible degree for the happy perpetuation of both - came about by a never-ending series of chance strikes and counterstrikes wrought by accidentally changing genes simply should not be accepted by the thinking public. The whole idea is madness, sheer madness. Only a Supreme Master Mathematician could possibly work it all out. Nature is filled with more mystery and intrigue than Agatha Christie could ever have dreamed of in her wildest flights of imagination. This truism comes as no surprise to those who recognize the Creator's infinite imagination and intelligence. Not everybody sees the hand of God in the amazing subculture we call parasitism. Janice Moore expresses her doubts: I was surprised to find the Plagiorhynchus-isopod association cited in the Creation Research Society Quarterly as one "evidence of design,". I can understand how parasite-induced behavioral alterations would interest a deity, and I am pleased to be in such good company, but if P. cylindraceus was indeed especially created, that must surely seem a perversion to a starling or isopod (2002, p. 43). Her reason for objecting to the concept of divine creation, if intended seriously, seems strikingly out of character with the eminent degree of intelligence displayed throughout the rest of her work. If God is not concerned about oxen (1 Cor. 9:9), how much less does He worry Himself with the lives of starlings and pillbugs, both of which plague the human race. Though He monitors the birth and death of every animal (Matt. 10:29), He deems them of no intrinsic value. They are, after all, merely "metabolizing computers". Their greatest value comes from the witness they provide to the genius of God. |
|
Notes 1The course of development from the fertilization of the egg to the production of a new generation of reproductive germ cells. In many animal species (such as butterflies) the cycle includes one or more intermediate larval phases in addition to the sexually-reproducing adult phase. Some plants, such as ferns, also have two distinct, independent stages in their life cycles. 2An upstream host is one in which a parasite lodges before transmission occurs and a downstream host is the one after . The upstream host is the host from which the parasite is coming and the downstream host is the one to which the parasite is going (Combes 1991, p. 868). 3The movement of an organism in a particular direction in response to an external stimulus such as gravity References Ackerman, Jennifer October 1997, Parasites: Looking for a Free Lunch, National Geographic Adamo, S. A. 2002, Modulating the modulators: parasites, neuromodulators and host behavioral change, Brain, Behavior and Evolution, 60:370-377 Attenborough, David 1990, The Trials of Life, Collins/BBC Books, London Bernays, E. A., and Singer, M. S. 2005, Taste alteration and endoparasites, Nature, Vol. 436, No. 7050, p. 476 Biron, D. G., Marché, L., Ponton, F., Loxdale, H. D., Galéotti, N., Renault, L., Joly, C., and Thomas, F. 2005, Behavioural manipulation in a grasshopper harbouring hairworm: a proteomics approach, Proceedings of the Royal Society B, 272, 2117-2126 Brodeur, J., and McNeil, J. Seasonal Microhabitat Selection by an Endoparasitoid Through Adaptive Modification of Host Behavior, Science, 1989, 244:226-228 Brown, S. P., Loot, G., Grenfell, B. T., and Guégan, J. F. 2001, Host manipulation by Ligula intestinalis : accident or adaptation?, Parasitology, 123 : 519-529 Combes, C. 1991, Ethological Aspects of Parasite Transmission, American Naturalist, Vol. 138, pp. 866-880 Denton, Michael 1985: Evolution: A Theory in Crisis, Adler & Adler, Bethesda Eberhard, W. G. 2000, Spider manipulation by a wasp larva, Nature, 406: 255-256 Hamilton, W. D. 1980, Sex versus non-sex versus parasite, Oikos, 35:282-290 Hurd, H., Warr, E., and Polwart A. 2001, A parasite that increases host lifespan, Proceedings of the Royal Society of London, Series B, 268: 1749-1753 Maitland, D. P. 1994, A parasitic fungus infecting yellow dungflies manipulates host perching behaviour, Proceedings of the Royal Society of London, Series B, 258: 187-193 Moore, J. 2002, Parasites and the Behavior of Animals, Oxford University Press, Oxford Moore, J. May 1984, Parasites That Change the Behaviour of Their Host, Scientific American Ponton, F., Lefevre, T., Lebarbenchon. C., Thomas, F., Loxdale, H. D., Marché, L., Renault. L., Perrot-Minot, M. J., and Biron, D. G. 2006, Do distantly related parasites rely on the same proximate factors to alter the behaviour of their hosts?, Proceedings of the Royal Society B, 273: 2869-2877 Poulin, R. 1995, "Adaptive" Changes in the Behaviour of Parasitized Animals: A Critical Review, International Journal for Parasitology, Vol. 25, No. 12, pp. 1371-1383 Rennie, J. January 1992, Living Together, Scientific American Thomas F., Schmidt-Rhaesa, A., Martin, G., Manu, C., Durand, P. and Renaud, F. 2002, Do hairworms (Nematomorpha) manipulate the water seeking behaviour of their terrestrial hosts?, Journal of Evolutionary Biology, 15: 356-361 Thomas, F., Adamo, S., and Moore, J. 2005, Parasitic manipulation: where are we and where should we go?, Behavioural Processes, 68: 185-199 Thomas, F., Renaud, F., and Poulin R. 1998, Exploitation of manipulators: "hitch-hiking" as a parasite transmission strategy, Animal Behaviour, 56: 199-206 Tierney, J. F., Huntingford, F. A., and Crompton, D. W. T. 1993, The relationship between infectivity of Schistocephalus solidus (Cestoda) and anti-predator behaviour of its intermediate host, the three-spined stickleback, Gasterosteus aculeatus, Animal Behaviour, Vol. 46, no. 3, pp. 603-605 Valentine, J. W. 2004: On the Origin of Phyla, The University of Chicago Press Yanoviak, S. P., Kaspari, M., Dudley , R., and Poinar, G. Jr . A pril 2008, Parasite-Induced Fruit Mimicry in a Tropical Canopy Ant, The American Naturalist, V ol. 171, No. 4: 536-544 Watanabe, M. 2004, Strepsipterans: Parasitic Insects That May Help Eradicate Pests, BioScience, May 2004 / Vol. 54 No. 5 Hughes, D. P., Wappler, T., and Labandeira, C. C. 2010, Ancient death-grip leaf scars reveal ant-fungal parasitism, Biology Letters, published online 18 August 2010, doi: 10.1098/rsbl.2010.0521 Beani, Laura 2006, Crazy wasps: when parasites manipulate the Polistes phenotype, Ann. Zool. Fennici, 43: 564-574
|
|
Dawn to Dusk publications |
Other printed material |
On the Web |
|
|
|
|
| Edited and expanded copies of this article, in reprint pamphlet form, can be purchased by going to the reprints order page. As well as reprints, Dawn to Dusk offers books in printed form and on CD-ROM. We mail to anywhere in the world! For more information on what is available, prices, and how to order, click the icon. |